Berlin, Germany (Weltexpress). The article combines existing information with recent independent analyses to provide new perspectives on bit-related welfare problems. In a study comparing bitted with bit-free behaviour in 66 horses ridden recreationally, 69 aberrant behaviours were identified as being bit-induced. Removal of the bit reduced the number of pain-induced behaviours by 87%. Bitted horses ridden competitively exhibit similar pain-induced behaviours, together with clinical signs consistent with bit-induced airway obstruction. Anatomical, physiological and aerodynamic evidence is presented supporting the proposition that “bleeding” in the racehorse is a bit-induced disease, analogous to a potentially fatal airway obstruction in man; negative pressure pulmonary oedema. The evidence suggests that once bit-free competition is allowed, many diseases currently classified as of unknown cause will be revealed as being caused by a combination of bit-induced pain and airway obstruction. The list includes dynamic collapse of the upper respiratory tract, dorsal displacement of the soft palate, epiglottal entrapment, cervical trachea deformity, exercise-induced pulmonary haemorrhage, exercise-induced arterial hypoxaemia, recurrent laryngeal neuropathy, premature exhaustion, breakdowns, catastrophic accidents and sudden death. The benefits of pain-free horsemanship cannot be made available until bit-free competition is allowed. The primary objective of the article is to provide access to the foundation evidence for the above propositions. A wide range of pain-free headstalls are available and a minimal-touch rein can provide non-invasive signals to the horse’s chin and bridge of nose. In the last 20 years, a burgeoning bit-free movement has shown that bit-free horsemanship improves horse welfare and advances equestrian safety. Bit-induced pain is reason enough for repealing bit-mandated rules in all disciplines and for reconsidering the implicit requirement for bit usage in racing.
Summary supplement: Interest in bit-free horsemanship was awakened in 2000 and constitutes a two-decade ‘natural experiment’ with a reassuring record on safety. Data comparing bitted and bit-free behaviour on 4 horses were published in 2009, with data on 66 horses in 2018. Statistical analysis of both data sets documents the positive welfare benefits of bit-free horsemanship and the negative welfare effects of bit usage. In 2017, an independent review of breathing, breathlessness and bridles concluded that the problem of bit-induced mouth pain in horses should be addressed. Bit-free horsemanship is now endorsed in many journals, books and videos. The Royal Dutch Equestrian Federation approves bit-free dressage at all levels short of Grand Prix. For many years, bit-free virtual dressage has been offered by the World Association of Western Dressage and by North American Western Dressage. In 2019, the Five Domains Model for animal welfare assessment applied to horses led to the adoption of Thoroughbred Welfare Assessment Guidelines in New Zealand. In 2020, these same guidelines were incorporated by the International Federation of Horseracing Associations in their Minimum Horse Welfare Standards. In the last 20 years, the general public’s concern about horse welfare has been increasingly expressed; questioning equestrian sports’ social licence to operate. A World Bitless Association has been formed. In sum, bit usage has been shown to be harmful to the horse. As equipment for racing is approved at the discretion of the stewards, it is suggested that this provides a path for bit-free racing to be introduced one horse at a time.
Keywords: horse,pain,conflict behaviour, welfare, horse-human relations, equitation science, E-BARQ, horse racing, breakdowns, catastrophic accidents, poor performance, asthma, learned helplessness, headshaking, bolting, bucking, rearing, ridden lameness, suffocation, exercise-induced pulmonary haemorrhage, EIPH, exercise-induced arterial hypoxaemia, “bleeding,” sudden death,
Prologue
Since before the Bronze Age, horsemanship has been handicapped by a prevalent view that a bit controls a horse. But because a bit often causes pain, this alone should be reason enough for discontinuing its use. In addition, this would remove a barrier to testing the hypothesis that a bit also causes airway obstruction. By drawing together the evidence on bit-induced pain and airway obstruction in one article, it is shown how working towards ending bit usage in equestrian sport could resolve both these welfare problems and their consequences.
“… it was a nasty thing! Those who have never had a bit in their mouths cannot think how bad it feels”
(Anna Sewell, Black Beauty, 1877).
1. Introduction

To avoid being cheated when buying a horse, Xenophon noted in 362 BC that “the way in which he lets you put the bit into his mouth, and the headpiece about his ears, should not escape you. This would be least likely to pass unnoticed if the bridle were put on and taken off in the sight of the purchaser.” (Morgan 1962). In a comparative study of 69 behaviours in 66 horses (Cook and Kibler 2018), “difficulty in bridling” was the fifth most common unwanted behaviour, occurring in 62% of the horses when bitted and 6% when bit-free. Similar bit-indicting data were reported for 68 other unwanted behaviours. In order of decreasing prevalence, the four most common behaviours were “hates the bit”, “fright”, “stiff necked” and “loss of control”; contrary to the myth that a bit controls a horse.
Clearly, Xenophon was familiar with the horse’s aversion to the bit. Nevertheless, he recommended that a rider should own two bits, both of a design we now call jointed snaffles. The canons of each were “rough” but in one the roughness was also “sharp.” In addition, small or large discs were added to discourage the horse from “grabbing the bit.” On the teeth being clenched, large discs would press into the tongue and roof of mouth to “make him keep his jaws apart and drop the bit.” In a note, Morgan adds, “… we see why the horse is represented with his mouth open in nearly all Greek works of art.” As another option, Xenophon recommended that, “little rings are hung from the joints of the bit in the middle …” Since then, many other designs have been developed. However, the basic concept of the bit, implicit in all its many versions, as a device whereby man has sought to dominate the horse by inflicting pain, has remained unchanged. The use of two bits has become standard practice. Notably, Bedouin horsemen did not use bits.
The pathophysiological effect of the bit was not described until quite recently (Cook 1999). That said, it had taken me an unconscionable time to recognize this effect. As a rider, I had always used a bit. As a veterinarian since 1952, even though my research was focused on diseases of the horse’s ear, nose and throat, I had been “blind’ to the bit for 47 years. Thanks to serendipity, this changed. The reader of a book I had published on airflow factors in the racehorse (Cook 1993) encouraged her dressage trainer, Allan Buck, to contact me. Buck was recommending the use of an unfamiliar bit-free bridle. I tested the bridle, a crossunder, on a 5-year-old, off-the-track racehorse with behavioral problems and liked it on this horse and, subsequently, all others. These first bit-free revelations prompted me to pose the question, “What does a bit do to a horse?”
Realizing, four years post-retirement, that the answer was “enormous harm”, I resolved to make the crossunder bridle commercially available. In the period 2000 to 2016, I marketed c.100,000 before retiring for the second time. During those years, crossunder bridles were adopted by riders of all ages and experience, in many countries, with horses of all types and ages, in a variety of terrains, in all weathers and for many disciplines. In monitoring this natural experiment for 16 years, I was not notified of any accident attributed to a horse being bit-free. On the contrary, I received accounts of incidents that might have resulted in accidents if the horse had not been bit-free.
Over the last 20 years, a vigorous bit-free movement has grown with recreational riders worldwide using bit-free bridles of many designs, both old and new. I now hypothesize that the bit is (1) the probable cause of many common diseases currently considered to be of unknown cause, (2) a handicap to performance, (3) a frequent but unacknowledged cause of loss of control and accidents and (4) a device that blocks the ultimate goal of equestrians to achieve rider-horse harmony. The hypotheses are eminently testable yet cannot be tested under competition conditions until bit use as standard practice is discontinued and bit-mandated rules are repealed.
In a parallel development over the same period, the general public has become increasingly concerned about horse welfare. I recommend that equestrian sport administrators collect and evaluate the evidence on bitted and bit-free horsemanship with a view to reconsidering their requirement for bit-usage. Stewards of racing could witness bit-free training trials and approve jockey/horse dyads for competing bit-free. Inaction could lead to equestrian sport losing its social licence to operate (Hampton et al 2020).
Campbell (2013) reviewed the responsibilities and ethical dilemmas that arise in equestrian sport and recommended that, in order to build a data base, governing bodies of all disciplines should develop compulsory systems for recording competition injuries. More recently, Campbell (2021) has cautioned that “Whilst the use of horses in sport continues to be accepted by the majority of the public, that social license is increasingly tenuous.” Campbell proposes the use of an ethical framework for critically assessing existing practices and making welfare-improving adjustments where necessary.
2. A Bit-Free Briefing
2.1 “Bleeding” in the racehorse
Twenty-seven years ago, using endoscopy to rule-out structures in the head as the location of the haemorrhage in what, at the time, was colloquially called “nose bleeding”, I proposed that the ‘blood’ seen at the nostrils was originating from the lungs (Cook 1974). With the introduction of fibreoptic endoscopes, this was confirmed (Pascoe et al 1981). Initially, it was widely suggested that ‘bleeding’ was an inherent and even a normal feature of the Thoroughbred. The name exercise-induced pulmonary haemorrhage (EIPH) was adopted. It was later proposed that EIPH is caused by an upper airway obstruction (Cook et al 1988). Since then, with added evidence, I have come to see that EIPH is bit-induced (Cook 2002, 2003) and analogous to an emergency during general anesthesia in man caused by airway obstruction, namely negative pressure pulmonary oedema (Deepika et al 1997, Baskhar and Fraser 2011, Cook 2014, 2016). A critique of the bit (Cook 1999) was followed by a book (Cook and Strasser 2003) in which it was noted that, when running at liberty, a horse’s lips are sealed and that use of a bit denies a horse this physiological necessity. Apart from causing pain, the presence of a bit prevents a horse from sealing its lips prior to running and therefore prevents a horse from creating the negative pressure in its oral compartments that is crucial to maintaining an unobstructed throat airway at exercise (see Figures 1-6 below).
2.2 Comparative studies of bitted/bit-free behaviour
Ashley et al (2005) drew attention to the paucity of pain research in the horse. In 2008, a pilot experiment at the Certified Horsemanship Association Conference, compared bitted and bit-free behaviour (Cook and Mills 2009). Quoting from the summary: “The study tested the null hypothesis that if a horse is ridden in a snaffle bridle and then a crossunder bridle, there will be no change in its behaviour.” It was predicted that there would be change and that behaviour would improve when bit-free. Four mature, riding-school horses, none of which had ever been ridden bit-free, were ridden through two consecutive, 4-minute dressage tests, first bitted then bit-free. An independent judge, Elizabeth (‘Mitzi’) Summers, marked the 27 phases of each test on a 10-point scale. The experiment was filmed, and the judge’s comments and scores recorded in real time. The results refuted the null hypothesis and upheld the prediction. Mean rider score when bitted was 37 and when bit-free 64. A binomial probability distribution indicated that the results were significantly different from random effects. All horses transitioned to the bit-free bridle unhesitatingly. Contrary to an experienced equestrian’s prediction, there were no out-of-control or ‘loose’ horses.
A six-page questionnaire based on 69 aberrant behaviours in 66 horses was completed twice by owners who transitioned their horse from bitted to bit-free (Cook and Kibler 2018). Again, quoting from the summary, “After mostly multiple years of bit usage, the time horses had been bit‐free ranged from 1 to 1095 days (median 35). The number of aberrant behaviours exhibited by each horse when bitted ranged from 5 to 51 (median 23); when bit‐free 0 to 16 (median 2). The number of aberrant behaviours for the total population when bitted was 1575 and bit‐free 208; an 87% reduction.”
A survey of 66 Equus caballus mandiblesin four US Natural History Museums revealed a high prevalence of bit-induced bone and dental pathology (Cook 2011). Thus, “periostitis (painful bone spur formation) of the interdental space (bars of the mouth) was found in not less than 62% of the domestic hemimandibles. Erosion of enamel and dentine was found in 61% of the second lower premolars (Triadan 306 and 406). 88% of the domestic mandibles showed one or both lesions.” Free-roaming horses showed no such lesions.
2.3 The Five Domains Model for animal welfare assessment
For 26 years the Five Domains Model has provided a scientific foundation for welfare assessment in mammalian and other species, thereby allowing evaluation of factors that contribute to animals experiencing a wide range of negative or positive ‘mental’ (i.e., welfare) states (e.g., Beausoleil and Mellor, 2012, 2015a, Mellor et al 2015; Mellor and Beausoleil 2015a, 2020; Beausoleil et al 2016; Littlewood and Mellor 2016; Mellor 2016, 2017, Ledger and Mellor 2018; Sherwen et al 2018; Harvey et al 2020; Mellor and Beausoleil 2020; Mellor et al 2020). The Five Domains Model has been adopted as the basis for racehorse welfare management by Thoroughbred Racing New Zealand (Thoroughbred Welfare Assessment Guidelines 2019; Mellor and Burns 2020) and the International Federation of Horseracing Authorities (2020). The Model has also been used to examine the negative welfare impacts of a wide range of particular equine management and riding procedures (McGreevy et al 2018). Building on the well-established physiological and pathophysiological understanding of the human subjective experiences of breathlessness (Beausoleil and Mellor 2015b), parallel changes in horses were interpreted, cautiously, in a review of equine welfare during exercise, which focussed on breathing, breathlessness and bridles (Mellor and Beausoleil 2017). The authors concluded there was “potential for ridden [bitted] horses to experience three forms of breathlessness; unpleasant respiratory effort, air hunger and chest tightness” and that “… most horses exhibit clear behavioural evidence of aversion to a bit in their mouths, varying from the bit being a mild irritant to very painful. This in itself is a significant animal welfare issue that should be addressed”. A follow-up review of mouth pain in horses drew attention to independently reported evidence of bit-induced lesions, the severity of which could cause pain ranging from mild to severe (Mellor 2020a). It also outlined the cogent scientific bases for interpreting particular equine behaviours as caused by mouth pain (Mellor 2020a), finally concluding that “the negative experiences that are most responsible for welfare compromise include the pain itself, but also, related to this pain, potentially intense breathlessness, anxiety, and fear.”
2.4 Surveys of horse behaviour
Observation of 76 box-stalled horses – differing only by discipline (same living conditions, breed, sex) – showed that each discipline’s (bitted) work was linked to a different pattern of stereotypic behaviours (Hausberger et al 2009). Hockenhull and Creighton (2012) studied owner‐reported ridden behaviour in UK leisure horses, noting that the high prevalence of behavioural problems (91%) may indicate “significant rider safety and horse welfare concerns.”
A dissertation on the interaction between rider, horse, and trainer (Blokhuis 2020) included a comment on how riding aids are currently classified. “Natural aids include the rider’s voice, seat, leg and hand, while unnatural comprises anything used to enhance these – a whip, spurs, or gadgets such as martingales, and draw reins, all of which ‘force’ the horse to comply.” The comment exemplifies how the antiquity of the bit has influenced our thinking to the extent of it being accepted as ‘natural.’ Similarly, the bit was not included as one of the ‘artificial’ aids by Hockenhull and Creighton (2012). The bit received little attention in an assessment of the adverse impacts of husbandry, veterinary and equitation interventions on horse welfare (McGreevy et al 2018).
In 2020, as already noted, publications on acute and chronic bit-induced damage to the soft and hard tissues of the mouth were reviewed in the context of mouth pain in horses (Mellor 2020a). It was noted that 27 of the 33 publications upon which behavioural comparisons were made were by contributors who were independent of the bitted vs bit-free debate, as were all of c.250 YouTube videos of horses in these categories.
Bit-pain behaviour is rarely recognized as such, being widely mistaken for normal behaviour of the species (Mellor and Beausoleil, 2017, Mellor 2019, 2020a, b, c, Leander 2020, Pearce et al, 2020, Bergmann 2020). An international survey of equestrians’ values and beliefs with regard to risk perception in daily horse interactions was led by a person familiar with risk assessment in the workplace and the concept of fitness for purpose (Chapman et al 2020). The word ‘bit’ was not mentioned in the article. Clearly, the bit was not recognized as a risk factor. The pain of whipping has been overlooked until quite recently (McGreevy and Jones 2020). Perhaps the question of bit-induced pain would gain more traction if a bitted rein were to be regarded as a whip by another name.
Increasing concern by the general public has prompted a reconsideration of equine welfare (Hampton et al 2020). An Equine Behaviour Assessment and Research Questionnaire (E‐BARQ) has been developed to obtain quantitative data on the domestic equine triad; training, management and behaviour (Fenner et al, 2020). The E-BARQ responses totaled 1584 and incorporated 268 behavioral items. Specific behaviours (e.g., poor tolerance of restraint and loading problems) during common equestrian activities in horses with a bitted bridle were associated with rearing, bucking and bolting (Romness et al, 2020). Again, my experience is that all three behaviours can be bit-induced.
Using a 24-item behavior-pain ethogram to study ridden-horse performance, it was concluded that “horses can pass an in-hand inspection, but show gait abnormalities when ridden, highlighted by behavioural changes” (Dyson and Ellis 2020). Twenty of the 24 behaviours, independently selected in the study were part of the 69 behaviours previously identified (Cook and Kibler 2018). All 24 are consistent with bit-induced behaviour.
Data from a video review of 147 Grand Prix dressage performances indicated that 68% of the horses exhibited an open mouth and 67% had their heads behind the vertical (Dyson and Pollard (2021). The authors noted, without specifically indicting the bit, that a potential reason was pain induced by tack. Both behaviours are consistent with being caused by the bit.
2.5 Advances in bit-free human-to-horse communication
Thanks to the telephone, email and one meeting, I have – for 15 years – enjoyed productive discussions with Dr Fridtjof Hanson in New Zealand, a retired cardiovascular surgeon and lifelong horseman. One outcome has been that Hanson explored the remarkable efficacy of the (bit-free) Bedouin bridle (Hanson and Cook 2017). Taking a lead from the platted rein of a Bedouin bridle and using a mountaineer’s kernmantel rope in conjunction with a bit-free headstall and noseband, Hanson subsequently developed a rein that provides proprioceptive signals to the horse’s whiskered chin, together with gentle pressure – if required – to the bridge of the nose (Hanson 2019). Simple in concept, such a rein can be assembled by the rider. We recommend this “whispering-to-the-whiskers” rein-aid for testing in all disciplines.
2.6 The bitted horse’s plight
Anatomically, the pathways for the respiratory and digestive systems intersect at the pharynx (Figure 1). Neurologically, the many reflexes that support each system are also at cross-purposes. In the running horse, a bit may trigger digestive system reflexes, e.g., chewing, salivation and swallowing rather than the respiratory reflexes required. When running a horse should not be salivating.
The horse’s tongue is a sense organ and muscular hydrostat, occupying the oropharyngeal sections of the digestive tract. Whatever its shape, its volume is constant. Compressed dorsoventrally by food, bit or tongue-tie, it compensates by bulging laterally. Retracted at its apex, it bulges at its root, obstructing the airway.
At liberty, one swallow with sealed lips prior to running evacuates air, saliva and food from the oral compartments, and – by creating a partial vacuum – causes the soft palate along the whole of its length to adhere to the root of the tongue and, at the soft palate’s elastic ‘button-hole’, to the ‘button’ of the larynx. The mechanism is that of a suction cup. For as long as the lips remain sealed and the tongue, jaw and larynx remain relatively immobile, this secures and maximizes the diameter of the nasopharyngeal airway by stabilizing the soft palate, allowing the horse to breathe freely while running. Thus, a horse at liberty runs with head and neck extended, lips sealed, teeth clenched, mouth dry, and the throat airway stable, confluent and unobstructed (Figures 1-6). None of these life-critical requirements are available to a bitted horse. A bit flexes the head and neck, breaks the lip seal, dissipates the digestive tract compartments’ vacuum, and mobilizes the tongue, jaw and larynx.
An understanding of these phenomena is facilitated by the explanation of each one in the captions for Figures 1 to 6.
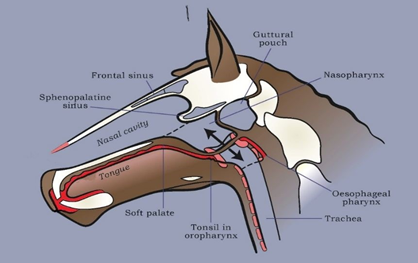
Figure 1. Bit-free and airway unobstructed during fast exercise. Head and neck extended; lips sealed; no air in digestive tract compartments (regions in red indicating the existence of a negative atmospheric pressure); soft palate adherent to root of tongue and securely ‘buttoned-up’ around the laryngeal ‘button’. Poll extension at fast exercise stretches the soft-walled nasopharyngeal airway longitudinally, providing a ‘tautness’ that resists the negative pressure collapsing forces of inspiration.
Key: white = bone; brown = soft tissue; pink = cartilage; red = digestive tract compartments (i.e., oral cavity, oropharynx, laryngeal pharynx and oesophageal pharynx)

Figure 2. Sealed lips and a dimple in the cheek of a running horse at liberty; visual evidence of a negative atmospheric pressure in the oral compartments at exercise. The dimple is the elliptical, dark shadow, starting just behind and slightly above the corners of the mouth. Its straight edge illustrates the partial vacuum in the buccal cavity causing the cheek to be sucked-in against the roof of the mouth at the level of the interdental space (“bars” of the mouth). For the same reason, the ventral edge of the molar arcade is also discernible.
[Screen shot from a video insert in a YouTube video for which a photo credit was not published]
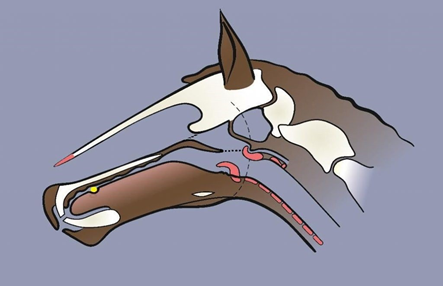
Figure 3a. Illustrating bit-induced dorsal-displacement of the soft palate (DDSP) during exercise. The lips are unsealed and the soft palate ‘unbuttoned.’ The short-dotted line shows severe obstruction of the airway at the posterior nares (choanae). The long-dotted line shows the elastic-sided ‘buttonhole’ of the soft palate (Figure 3b) released from the epiglottal ‘grommet’, further compromising the nasopharyngeal airway. This event, occurring during a race, could cause a horse to suffocate (‘choke-up’), collapse and even die, with or without any jockey-reported sound of respiratory distress. Instability of the soft palate (i.e., a ‘loosening’ of palato-laryngeal adhesion without any ‘unbuttoning’) is inevitable with loss of the lip-seal in a bitted horse. Poiseuille’s law determines that even the slightest degree of suffocation, as during any ‘loosening’, is of relevance to athletic performance.
Key: Yellow = the bit in the ‘over-tongue’ position
Figure 3b.
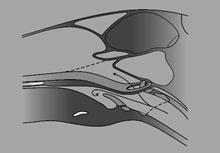
A perspective view of the nasopharyngeal airway during dorsal displacement of the soft palate as in Figure 3a, illustrating the anatomy of the soft palate ‘buttonhole’ (ostium intrapharyngium). The forked arrow shows how airflow during expiration might cause the soft palate to flutter like a wet blanket in the breeze; as when a racehorse ‘chokes-up’ with a gurgling respiratory noise similar to that which, in human medicine, is known colloquially as a ‘death-rattle.’ The ‘button-hole’, being elastic in nature, shrinks when released from the retaining ‘grommet’ of the epiglottis, further obstructing airflow.
Figure 4.

obstruction of the airway during exercise from the effects of unsealed lips and poll flexion. This and further degrees of bit-induced head and neck flexion (e.g., nasal plane behind the vertical) increases the work of breathing and, as determined by Poiseuille’s law, will – it is proposed – result in pulmonary barotrauma (bruising of the infinitely delicate and lace-like alveoli), i.e., ‘waterlogging’ and ‘bleeding’. Additional ‘throttling’ occurs from dynamic collapse of the nasopharyngeal airway at each inspiration, as indicated by the arrows. Such changes could trigger pharyngeal or laryngeal reflexes (i.e., ‘gag’ reflexes or laryngospasm). The triggering of a trigemino-cardiac reflex could conceivably cause heart failure and sudden death.
Figure 5:
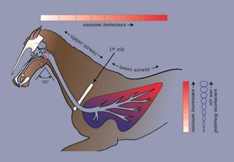
Postulated mechanism for the cause of ‘bleeding’ in the racehorse, i.e., bit-induced airway obstruction leading to negative pressure pulmonary oedema (NPPO). With a bitted racehorse being ‘rated’ (rein tension causing poll flexion), the lips will be unsealed, pressure in the oral compartments will be atmospheric and there will be loss of adhesion of the soft palate to the root of tongue and larynx. At each rapid inspiration when galloping, the soft palate and roof of the nasopharynx will undergo dynamic collapse, further obstructing the nasopharyngeal airway. Pulmonary barotrauma will, it is proposed, result in heavily blood-stained fluid being drawn into the interstitial tissues of the lung, the alveoli and small airways at each intake of air. Some of this fluid may be seen at the nostrils during or after a race but the more serious effect will be to quickly change the nature of a healthy lung from an infinitely light and fluffy ‘sponge’ into a heavy, soggy ‘pudding.’ A law of physics (Poisseuille’s law) determines that the most serious ‘waterlogging’ will occur in the caudo-dorsal regions of the lung (redder in the diagram), because inspiratory negative pressure increases with distance from the obstruction. Another principle of the law (but not depicted in the diagram) determines that dynamic collapse of the cervical trachea will become increasingly severe along the course of the neck, being most severe at the level of the first rib, as explained in Figure 6a. Any pre-existing degree of left recurrent laryngeal neuropathy (highly likely) will add to the airway obstruction.
Figure 6a
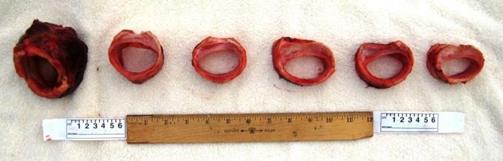
‘Scabbard’ trachea deformity in a Thoroughbred racehorse: From left to right, transverse sections through the cricoid cartilage and cervical tracheal rings 1, 9, 18, 27 and 36 show caudally progressive deformity as determined by Poiseuille’s law resulting, it is proposed, from repeated episodes of nasopharyngeal airway obstruction.
Figure 6b.
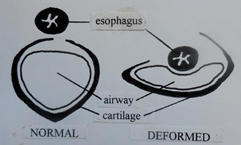
Comparing the cross-sectional shape of a normal cervical tracheal ring (lumen approximately circular) with that of a caudal ring permanently deformed (lumen elliptical) by, it is postulated, recurrent episodes of dynamic collapse of its pliant dorsal membrane, sequential to bit-induced airway obstruction at the level of the nasopharynx. In my experience, this is a common defect and one that may be overlooked at autopsy when the trachea is routinely opened by longitudinal incision of the tracheal rings’ dorsal membranes
2.7 Comparative evidence on negative pressure pulmonary oedema in man
A consensus statement on EIPH by the American College of Veterinary Internal Medicine concluded with a “Strong recommendation that EIPH be considered a disease” (Hinchcliff et al 2016). Though this statement was itself a step forward at the time, the cause was not addressed. In man, airway obstruction is a recognized cause of negative pressure pulmonary oedema, a disease which, in the author’s opinion, is analogous to EIPH in the horse, a view further developed by Mellor and Beausoleil (2017). Several case reports of equine pulmonary oedema following acute airway obstruction are relevant (Kolias-Baker et al 1993, Tute et al 1996) for comparison with similar emergencies in man (Deepika et al 1997).
Exercise-induced arterial hypoxaemia is recognized in the horse (Manohar et al 1985). Again, the cause is classified as ‘unknown’ but my opinion is that this may be yet another effect of bit-induced airway obstruction.
2.8 Bit-induced pain
Bit use causes pathophysiological changes during exercise (Cook 1981,1982,1999,2000,2003,2014,2016,2019a,2019b,2020; Cook et al 1988; Cook and Strasser 2003; Mellor and Beausoleil 2017; Mellor 2020a). The airway will often be partially obstructed, so that a bitted horse at exercise draws breath with difficulty while also experiencing mouth pain. The interface of bit-on-bone is out of sight and pain is difficult to quantify but three tests enable equestrians to assess for themselves what a bitted horse might experience (Mellor 2020a, b). Similarly, a breathing test is described, enabling riders to sense what a nose-breathing horse might experience when trying to breathe with unsealed lips (Cook 2019b).
The potential for bit-induced pain (Mellor 2020a) is illustrated by considering bit-impacted tissues (Figures 7-9).
Bit-on-bone (i.e., bit pressure on periosteum) and bit-on-mandibular teeth is illustrated in Figure 7. Bit pressure will stimulate pain receptors in interdental periosteum and in the first premolars on both sides of the jaw (Cook 1999, Cook and Strasser 2003, Mellor 2020a). It will also bilaterally stimulate receptors associated with unerupted or erupted ‘wolf’ teeth in front of the premolars and in the long, horizontally aligned, reserve crown and root of the permanent canines (Figure 8). In addition to acute contact-pain in these locations, bit-induced injury leads to what has to be painful bone spur formation at the interdental space (‘bars’ of the mouth). Repeated contact also causes erosion of premolar teeth. Both of these are likely to increase pain intensity with further bit contact, i.e., the development of hypersensitivity to the bit (Cook 2011, Mellor 2020a).
With the tongue over the bit, the lateral sections of a bit’s mouthpiece may, with ventral movement of the mouthpiece, pinch the tongue between the bit and the knife-edge ‘bars’ of the mouth or directly stimulate the mental branch of the mandibular nerve (Figures 7-9).
Figure 7.

‘Bit-on-bone’ contact of a double bridle at the interdental space. The tongue is frequently retracted behind the bit or placed over it. An exquisitely sensitive organ, the tongue, even when it lies under the bit, does not serve as a cushion for protecting the bars. The multiple strategies adopted by a horse to avoid the pain of a bit, too often thought of as “unwanted behaviours” and “vices”, speak for themselves.
Figure 8.
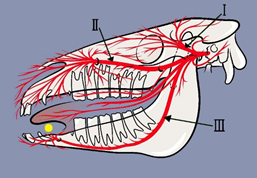
Distribution of the three facial branches of the Trigeminal nerve (Cranial V); the ophthalmic (I), maxillary (II) and mandibular (III) branches. The bit is shown in the tongue-over-bit position. Note the presence of an erupted ‘wolf’ tooth (vestigial first premolar) in the upper jaw and the common but overlooked occurrence of an unerupted ‘wolf’ tooth in the lower jaw (Sisson and Grossman 1938). Not shown but highly relevant, the mandibular branch carries both sensory and motor fibres and has complex connections with the Facial nerve (VII) and the Glossopharyngeal (IX), being itself interlaced with myelinated sympathetic fibres from the Vagus (X) (Sisson and Grossman 1938). As a result, the mandibular branch is the pathway for three large groups of life-critical protective reflexes, the oral-pharyngeal reflexes, the laryngeal reflexes and the trigemino-cardiac reflexes (Chowdury et al 2015, Miller 2002).
Figure 9.

Transverse section of the interdental space (‘bars’ of the mouth), just caudal to the mental foramen, of an above-average size horse compared with a standard-size hen’s egg. Red beads represent fibers of the mandibular nerve, also known as the “mental nerve.”
Discussion
In all species, eating and breathing are life-critical functions. The mammalian nervous system has evolved major resources for these functions. A wealth of sensory receptors for motor reflexes are located in the oral cavity and upper respiratory tract. A review of these mammalian oral and pharyngeal (aero-digestive) reflexes lists their complexity and emphasizes the pivotal role of the tongue (Miller 2002). Trigeminocardiac reflexes add a further layer of complexity (Chowdury et al 2015). All such reflexes are relevant to the fact that horses are obligatory nose-breathing animals, yet required by bit-mandated rules and bit-implicit standards to carry out high intensity physical work with a foreign body in their mouth, making nose-breathing difficult. A horse’s inability to mouth-breathe renders it especially vulnerable to nasopharyngeal airway obstruction.
A bit has the capacity to trigger noxious stimuli via the entire mandibular branch of the trigeminal nerve and to do this simultaneously, persistently, recurrently and bilaterally. The defensive array of reflexes in a horse’s mouth and upper airway highlights the potential for bit use to have a wide range of disruptive effects.
Unlike a rat in a Skinner box experiment, a bitted horse does not have the option of learning to press a lever to dismiss pain. What a bitted horse does learn are multiple strategies for trying to evade the bit, none of which are ultimately successful. In regard to the terminology of learning-theory, the concept of bit-induced ‘pressure’ (a euphemism for pain), even when followed promptly by ‘release’ (at best, a momentary cessation of pain) is not, in my opinion, negative reinforcement but positive punishment.
Conclusions
Bitted rules and standard practices have the effect of both causing harm and, because bit-free comparison is ruled-out, shielding the enormity of that harm from being discovered. Yet there is ample evidence that a bit causes a horse pain. This alone is reason enough for discontinuing its use in all disciplines. There is, in addition, sufficient evidence to infer that a bit suffocates and, in a racehorse particularly but not exclusively, triggers a pathophysiological cascade of negative pressure pulmonary oedema, catastrophic accidents and sudden death. Testing of the inference awaits action on the pain-based recommendation.
Conflict of interest
None to declare
Funding
Preparation of the article was self-funded
Ethical approval
None required
References
- Ashley, F.H; Waterman-Pearson, A.E.; Whay, H.R (2005): Behavioural assessment of pain in horses and donkeys: application to clinical practice and future studies. Equine Vet. J., 37, 565-575.
- Beausoleil, N.J. and Mellor, D.J. (2012): Complementary roles for systematic analytical evaluation and qualitative whole animal profiling in welfare assessment for Three Rs applications. ALTEX Proceedings 1 (WC8), 455-460.
- Beausoleil, N.J. and Mellor, D.J. (2015a): Advantages and limitations of the ‘Five Domains’ model for assessing animal welfare impacts associated with vertebrate pest control. New Zealand Veterinary Journal 63, 37-43.
- Beausoleil, N.J. and Mellor, D.J. (2015b): Introducing breathlessness as an animal welfare issue. New Zealand Veterinary Journal, 63, 44-51.
- Beausoleil, N.J., Fisher, P., Littin, K.E., Warburton, B., Mellor, D.J., Dalefield, R.R. and Cowan, P. (2016): A systematic approach to evaluating and ranking the relative animal welfare impacts of wildlife control methods: Poisons used for lethal control of brushtail possums (Trichosurus Vulpecula) in New Zealand. Wildlife Research , 43(7), 553-565. http://dx.doi.org/10.1071/WR16041.
- Bhaskar, B.; Fraser, J.F. (2011): Negative pressure pulmonary oedema revisited: Pathophysiology and review of management. Saudi J. Anaesth. 5, 308–313.
- Bergmann, I.M. (2020): Naturalness and the legitimacy of Thoroughbred racing: A photo-elicitation study with industry and animal advocacy informants. Animals , 10(8), 1513; https://doi.org/10.3390/ani10091513
- Blokhuis, M.Z. (2020): Interaction Between Rider, Horse and Equestrian Trainer – A Challenging Puzzle. Södertörn Doctoral Dissertation, Södertörn University, Stockholm, Sweden. Available online at: https://www.diva-portal.org/smash/get/diva2:1303083/FULLTEXT01.pdf [Accessed 11/18/20]
- Campbell, M. (2013): The role of veterinarians in equestrian sport: A comparative review of ethical issues surrounding human and equine sports medicine. Vet J. 13,198, 535-540.
- Campbell, M (2021): An Ethical Framework for the Use of Horses in Competitive Sport: Theory and Function Animals, 11(6), 1725; https://doi.org/10.3390/ani11061725
- Chowdhury, T.; Mendelowith, D.; Golanov, E.; Spiriev, T.; Arasho, B.; Sandu, N.; Sadr-Eshkevari, P.; Meuwly, C.; Schaller, B. (2015): Trigeminocardiac reflex: The current clinical and physiological knowledge. J. Neurosurg. Anesthesiol. 27, 136–147.
- Cook, W.R. (1974): Epistaxis in the racehorse. Equine Vet. J. 5, 45-58.
- Cook, W.R. (1981): Some observations on form and function of the equine upper airway in health and disease, Part I: The pharynx. Proceedings of the 27th Annual Convention of the American Association of Equine Practitioners, p. 355-392 Nov.-Dec.
- Cook, W.R. (1982): The biomechanics of intermittent suffocation at exercise in the horse. Der Praktische Tierarzt 4, 288-294.
- Cook, W.R. (1988): Hypotheses on exercise-induced pulmonary hemorrhage in horses. J. Amer. vet. med. assoc. 193, 8-10. (Letter to editor)
- Cook, W.R. (1992): Questions theory on cause of exercise-induced pulmonary hemorrhage. J. Amer. vet. med. assoc. 201, 1661-1662.
- Cook, W.R. (1993): Specifications for speed in the racehorse; the airflow factors. The Russell Meerdink Company, Menasha, WI, USA.
- Cook, W.R. (1999): Pathophysiology of bit control in the horse. J vet. eq. sci., 19, 196-204.
- Cook, W.R. (2000): A solution to respiratory and other problems caused by the bit. Pferdeheilkunde,16, 333-351.
- Cook, W.R. (2002): Bit-induced asphyxia in the horse: Elevation and dorsal displacement of the soft palate at exercise. J vet. eq. sci. 22, 7-14.
- Cook, W.R. (2003): Bit-Induced Pain; a cause of fear, flight, fight and facial neuralgia in the horse. Pferdeheilkunde,19, 1-8.
- Cook W.R. (2008): Experimental assessment of horse and rider responses to changing from a bitted to a bitless bridle. The Certified Horsemanship Association International Conference; https://bitlessbridle.com/the-cha-experiment/ [Accessed online 15 December 2020].
- Cook, W.R, (2011): Damage by the bit to the equine interdental space and second lower premolar. Equine Vet. Educ. 2011, 23(7), 355-360. DOI:10.1111/j.2042-3292.2010.00167.x. [Accessed 11/10/20]
- Cook, W.R. (2013): An endoscopic test for bit-induced nasopharyngeal asphyxia as a cause of exercise-induced pulmonary haemorrhage in the horse. Equine Vet. J. 46, 256-257.
- Cook, W.R. (2014): A hypothetical, aetiological relationship between the horse’s bit, nasopharyngeal asphyxia and negative pressure pulmonary oedema. Equine Vet. Educ. 26 (7), 381-389.
- Cook, W.R (2015): Man bites horse. Horse Conscious ;https://www.horseconscious.com/equipment/man-bites-horse/ [Accessed online on 15 December 2020].
- Cook, W.R (2016): Bit-induced asphyxia in the racehorse as a cause of sudden death. Equine vet. educ. 28, 405-409.
- Cook, W.R. (2018): Unintended consequences in preparing the ‘big horse’ for the ‘big race’. Horsetalk, 9 Dec.; www.horsetalk.co.nz/2018 [Accessed online on 15 December 2020]
- Cook, W.R. (2019a): Horsemanship’s elephant in the room. Weltexpress 15 February ; https://en.weltexpress.info/2019/02/15/horsehumanships-elephant-in-the-room-the-bit-as-a-cause-of-unsolved-problems-affecting-both-horse-and-rider/ [Accessed online on 29 June 2020]
- Cook, W.R. (2019b): Man bites horse. Weltexpress, Germany September 8, https://en.weltexpress.info/2019/09/08/human-biteshorse/
- Cook, W.R (2020): Horse to man: an urgent message. The Horse’s Hoof, Fall Issue 80, pp 43-44.
- Cook, W.R. Williams, R.M.; Kirker-Head, C.A.; Verbridge, D.J (1988): Upper airway obstruction (partial asphyxia) as the possible cause of exercise induced pulmonary hemorrhage in the horse: a hypothesis. J. eq. vet. sci. 8,11-26.
- Cook, W.R; Strasser, H. (2003): Metal in the mouth: The abusive effects of bitted bridles. Sabine Kells, Qualicum Beach, BC, Canada.
- Cook, W.R.; Mills, D.S. (2009): Preliminary study of jointed snaffle vs. crossunder bitless bridles: Quantified comparison of behaviour in four horses. Equine Vet. J., 41, 827-830. https://doi.org/10.2746/042516409X472150 [Accessed online on 15 October 2020]
- Cook, W.R.; Kibler, M. (2019): Behavioural assessment of pain in 66 horses, with and without a bit. Equine Vet. Educ. 31(10), 551-560. doi.org/10.1111/eve.12916 [Accessed online on 29 June 2020]
- Deepika, K.; Barrocas, Q.M.; Fonseca, J.J.; Bikasi, G.B. (1997): Negative pressure pulmonary edema after acute upper airway obstruction. J. Clin. Anaesth., 9, 403–408. [CrossRef]
- Dyson, S.; Ellis, A.D. (2020): Application of a Ridden Horse Pain Ethogram to horses competing at 5-star three-day-events: Comparison with performance. Equine Vet. Edu. published online at https://doi.org/10.1111/eve.13415 (accessed 2 January 2021)
- Fenner, K.; Matlock, S.; Williams, J.; Wilson, B. (1982): Validation of the Equine Behaviour Assessment and Research Questionnaire (E-BARQ): A new survey instrument for exploring and monitoring the domestic equine triad. Animals,10(11), 2; https://doi.org/10.3390/ani10111982
- Hanson, E.F.F. (2019): The Positive Reinforcement Rein: Game changer and rule changer? The Horses Hoof, Issue 76;
- Hanson, E.F.F.; Cook, W.R. (2017): The rediscovery of the Bedouin Bridle: A welfare and safety enhancer. The Horse’s Hoof, Issue 60. http://en.weltexpress.info/2017/03/17/the-bedouin-bridle/ [Accessed online on 29 June 2020]
- Hampton, J.O.; Jones, B.; McGreevy, P.D. (2020): Social license and animal welfare: developments from the past decade in Australia. Animals,10(12), 2237; https://doi.org/10.3390/ani10122237
- Harvey, A.M., Beausoleil, N.J, Ramp, D. and Mellor, D.J. (2020): A ten-stage protocol for assessing the welfare of individual non-captive wild animals: Free-roaming horses (Equus ferus caballus) as an example. Animals,10(1),148; doi:10.3390/ani10010148
- Hausberger M, Gautier E, Biquand V, Lunel C, Je´go P (2009): Could Work Be a Source of Behavioural Disorders? A Study in Horses. PLoS ONE 4(10): e7625. doi:10.1371/journal.pone.0007625
- Hinchcliff, K.W. (2014): Exercise-induced pulmonary haemorrhage (EIPH). In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elsevier: New York, NY, USA; pp. 633–647.
- Hinchcliff, K.W.; Couetil, L.L.; Knight, P.K.; Morley, P.S.; Robinson, N.E.; Sweeney, C.R.; Van Ercj, E. (2015): Exercise induced pulmonary haemorrhage in horses: American College of Veterinary Internal Medicine consensus statement. J. Vet. Intern. Med, 29, 743–758
- Hockenhull, J.; Creighton, E. (2012): The use of equipment and training practices and the prevalence of owner-reported ridden behaviour problems in UK leisure horses. Equine Vet. J. 45(1) 15-19. doi.10.1111/j.2042-3306.2012.00567.x
- International Federation of Racehorse Associations Minimum Horse Welfare Standards (2020): https://www.ifhaonline.org/default.asp?section=Resources&area=0&story=1076 [Accessed on line 5 July 2020]
- Kollias-Baker, C.A.; Pipers, F.S.; Heard, D.; Seeherman, H. (1993): Pulmonary edema associated with transient airway obstruction in three horses. JAVMA, 202, 1116–1118. [PubMed]
- Lang, S.A.; Duncan, P.G.; Shephard, D.A.E.; Ha, H.C. (1990): Pulmonary oedema associated with airway obstruction. Can. J. Anaesth. 37, 210–218. [CrossRef] [PubMed]
- Leander, A. (2020): News and Views – A Bit of an Issue 1: New Zealand Equine Veterinary Association letter regarding Mellor, D. “Bit Blindness” in VetScript2020, 33(9), 32-34. VetScript,33(12) 5-6. VetScript is the Monthly Magazine of the New Zealand Veterinary Association.
- Ledger, R.A. and Mellor, D.J. (2018): Forensic use of the Five Domains Model for assessing suffering in cases of animal cruelty. Animals, 8(7) 101; doi:10.3390/ani8070101.
- Littlewood, K. and Mellor, D.J. (2016): Changes in the welfare of an injured working farm dog assessed using the Five Domains Model. Animals6(9), 58; doi:10.3390/ani6090058.
- Manohar, M; Goetz, T.E; Hassan, A.S. (1985): Effect of prior high-intensity exercise on exercise-induced arterial hypoxemia in Thoroughbred horses. J Appl Physiol. 90 (6):2371-7 doi: 10.1152/jappl.2001.90.6.2371
- McGreevy, P.; Jones, B. (2020): 10 reasons to stop whipping racehorses including new research revealing the likely pain it causes. The Conversation, 12 November; https://theconversation.com/10-reasons-to-stop-whipping-racehorses-including-new-research-revealing-the-likely-pain-it-causes-149271 [Accessed online on 15 December 2020]
- McGreevy, P.; Berger, J.; De Brauwere, N.; Doherty, O.; Harrison, A.; Fiedler, J.; Jones, C.; McDonnell, S.; McLean, A.; Nakonechny, L.; et al. (2018): Using the Five Domains Model to assess the adverse impacts of husbandry, veterinary, and equitation interventions on horse welfare. Animals 8, 41.
- Mellor, D.J. (2016): Updating animal welfare thinking: Moving beyond the “five freedoms” towards “A life worth living” Animals 6(3), 21; 2016 https://doi.org/10.3390/ani6030021 [Accessed on line on 1 December 2020]
- Mellor, D.J. Equine welfare during exercise 1 Do we have a bit of a problem, (2019): https://www.slideshare.net/SAHorse/equine-welfare-during-exercise-do-we-have-a-bit-of-a-problem. Also available at: https://www.youtube.com/watch?v=rY4yEC7lhco [Accessed on 29 June 2020]
- Mellor, D.J. (2020a): Mouth pain in horses: Physiological foundations, behavioural indices, welfare implications and a suggested solution. Animals,10(4), 572; https://doi.org/10.3390/ani10040572
- Mellor, D.J. Bit Blindness. (2020b): VetScript , 33(9), 32-34.
- Mellor, D. (2020c): News and Views – A Bit of an Issue 3: D. Mellor’s response to letters objecting to his Bit Blindness article [Mellor, D. VetScript, 33(9), 32-34]. VetScript 2020c, 33(12), 7.
- Mellor, D.J.; Beausoleil, N.J. (2017): Equine welfare during exercise: An evaluation of breathing, breathlessness and bridles. Animals, 7(6), 41; doi:10.3390/ani7060041 http://www.mdpi.com/2076-2615/7/6/41
- Mellor, D.J.; Burns, M. (2020): Using the Five Domains Model to develop Welfare Assessment Guidelines for Thoroughbred horses in New Zealand. N.Z. Vet. J. 68(3), 150-156. Available online at: https://doi.org/10.1080/00480169.2020.1715900 [accessed 15 March 2020].
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C (2020): The 2020 Five Domains Model: Including human–animal interactions in assessments of animal welfare. Animals, 10(10), 1870; doi:10.3390/ani10101870
- Mellor, D.J. and Beausoleil, N.J. (2020): Moving beyond a problem-based focus on poor welfare towards creating opportunities to have positive welfare experiences. In: Mental Health and Well-being in Animals, 2nd Edition (Ed. Franklin D. McMillan), CAB International, Wallingford, UK, Ch 5, pp 50-66.
- Mellor, D.J., Hunt, S. & Gusset, M. (eds) (2015): Caring for Wildlife: The World Zoo and Aquarium Animal Welfare Strategy. WAZA Executive Office, Gland, Switzerland, pp87. At: https://www.waza.org/wp-content/uploads/2019/03/WAZA-Animal-Welfare-Strategy-2015_Landscape.pdf
- Thoroughbred Welfare Assessment Guidelines (2020): https://loveracing.nz/OnHorseFiles/NZTR%20Thoroughbred%20Welfare%20Guidelines%202020%20Final.pdf (accessed 5 July 2021). New Zealand Thoroughbred Racing, Petone, NZ, 2019a
- Miller, A.J. (2002): Oral and pharyngeal reflexes in the mammalian nervous system: Their diverse range in complexity and the pivotal role of the tongue. Critical Reviews in Oral Biology and Medicine, 13(5); https://doi.org/10.1177/154411130201300505 Accessed 6/9/21
- Morgan, M.H. (1962): The art of horsemanship by Xenophon: Translated with chapters on the Greek riding-horse and with notes. J. A. Allen & Co, London
- Pascoe, J.R.; Ferraro, G.L.; Cannon, J.H.; Arthur, R.M.; Wheat, J.D. (1981): Exercise-induced Pulmonary Haemorrhage in racing Thoroughbreds: A preliminary study. Am. J. Vet. Res. 42, 703-707.
- Pearce, T.; Bell, J.; Illston, A. (20200: News and Views – A Bit of an Issue 2: Letter regarding Mellor, D. Bit Blindness [VetScript 2020, 33(9), 32-34]. VetScript 2020, 33(12), 6-7.
- Romness, N.; Fenner K.; McKenzie, J.; Anzulewicz, A.; Burattini, B.; Wilson, B.; McGreevy, P. (2020): Associations between owners’ reports of unwanted ridden behaviour and in-hand behaviour in horses, Animals,10(12), 2431. https://doi.org/10.3390/ani10122431
- Sewell, A. (1877): Black Beauty: The Autobiography of a Horse. Jarrold and Sons, Norwich, England.
- Sherwen, S.L., Hemsworrth, L.H., Beausoleil, N.J., Embury, A. and Mellor, D.J. (2018): An animal welfare risk assessment process for zoos. Animals, 8(8), 130; doi.org/10.3390/ani8080130
- Sisson, S.; Grossman, J.D. (1938): The Anatomy of the Domestic Animals. Third Edition. W.B. Saunders, Philadelphia and London.
- Tute, A.S.; Wilkins, P.A.; Gleed, R.D.; Credille, K.M.; Murphy, D.J.; Ducharme, N.G. (1996): Negative pressure pulmonary edema as a post-anesthetic complication associated with upper airway obstruction in a horse. Vet. Surg. 25, 519–523. [PubMed]



















